Transporter Drug Interaction Tools
At Krishgen Biosystems, we are committed to providing cutting-edge solutions for evaluating Transporter Drug Interactions through our collaborations with industry leaders ReadyCell and BioIVT. Understanding how drug candidates interact with transporters is crucial for predicting drug-drug interactions (DDIs), optimizing pharmacokinetics, and ensuring drug safety.

Krishgen, via partners, offers products for:
Efflux Transporter Studies
• P-glycoprotein (P-gp/MDR1) assays
• Breast Cancer Resistance Protein (BCRP) Assays
• Multidrug Resistance-Associated Proteins (MRPs) Assays
• Bile Salt Export Pump (BSEP) Assays
Uptake Transporter Studies
• Organic Anion Transporting Polypeptides (OATPs) Assays
• Organic Cation Transporters (OCTs) Assays
• Multidrug and Toxin Extrusion Transporters (MATEs) Assays
Blood-Brain Barrier (BBB) Studies
• Efflux Transporters (P-gp, BCRP) Assays
• Uptake Mechanisms via specific transporters
Drug-Drug Interaction (DDI) Studies
• Efflux and uptake transporter kits for identifying substrates or inhibitors.
• Comprehensive transporter interaction tools
Key Benefits
- Comprehensive and Innovative Assay Portfolio: Krishgen provides a wide range of transporter assays, enabling a thorough evaluation of transporter interactions.
- Regulatory Alignment: Assays are developed in accordance with guidelines from major regulatory authorities, facilitating data acceptance in regulatory submissions.
- Expertise in Transporter Research: Both companies offer deep expertise in transporter biology, providing valuable insights and high-quality data to support drug development decisions.
Applications
- Drug-Drug Interaction Studies: Identify potential interactions that may alter the pharmacokinetics of co-administered drugs.
- Regulatory Compliance: Generate data required by regulatory agencies to assess transporter-mediated interactions, ensuring adherence to guidelines.
- Pharmacokinetic Profiling: Understand the role of transporters in drug absorption, distribution, metabolism, and excretion (ADME) to optimize dosing regimens.
Key Features of ReadyCell Permeability Assays
High-Quality Epithelial Models:
ReadyCell's kits utilize fully differentiated, polarized epithelial cells, such as Caco-2 and MDCKII, to mimic the structure and function of intestinal and other epithelial barriers.
Physiological Relevance:
Models like CacoGoblet Kits integrate mucus-secreting HT-29 cells, providing a barrier that closely mimics the intestinal environment, enhancing relevance to in vivo conditions.
Scalability and Convenience:
Available in formats suitable for high-throughput screening, the kits support both small-scale R&D and large-scale preclinical studies.
Innovative Shipping Technology:
ReadyCell’s patented Shipping Medium® ensures that kits arrive with viable, ready-to-use cells, eliminating preparation delays and maintaining experimental reliability.
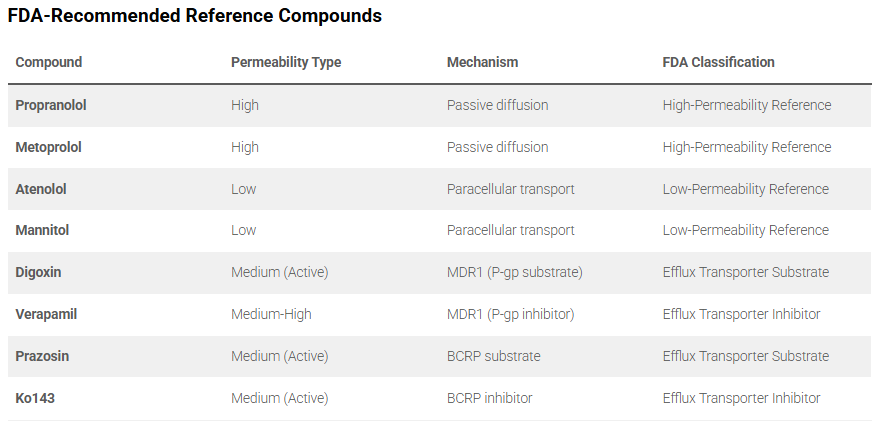
Permeability Assays Reference Compounds
Recommended reference compounds for permeability assays
Permeability assays are important for understanding how molecules cross biological barriers, assessing both their ability to cross and the mechanisms involved. While drugs are often absorbed by passive diffusion, some molecules can also be substrates for membrane proteins or transporters.
MATE1 transporter expression – PreadyTake MATE1 contains HEK293 cells transfected with the SLC47A1 gene to overexpress the drug and toxin transporter1 (MATE1). This is a membrane transporter of clinical importance for assessing drug-transporter interactions at preclinical stages.
OCT2 transporter expression – PreadyTake OCT2 contains HEK293 cells transfected with the SLC22A2 gene to overexpress organic cation transporter-2 (OCT2), a primarily renal uptake transporter expressed on the basolateral (blood) side of proximal tubule cells.
OATP1B3 transporter expression -PreadyTake OATP1B3 contains HEK293 cells transfected with the SLCO1B3 gene to overexpress the organic anion-transporting polypeptide 1B3 (OATP1B3), a membrane transporter of considerable clinical importance, to evaluate drug-transporter interactions in preclinical stages.
BCRP transporter expression – Our PreadyPort BCRP model contains MDCKII cells transfected with the ABCG2 gene to overexpress the breast cancer resistance protein (BCRP), a membrane transporter of considerable clinical importance, to evaluate drug-transporter interactions in preclinical stages.
MDR1 transporter expression – The multidrug resistance protein 1 (MDR1) also known as P-glycoprotein (P-gp) is a a membrane efflux protein which needs from the ATP hydrolysis to actively translocate substrates against an electrochemical gradient. They are present in our PreadyPort MDR1 plates.
Transporter-Certified™ Hepatocytes – TRANSPORTER CERTIFIED® hepatocytes are cryopreserved human hepatocytes that are validated to have in vivo-relevant transporter function under conditions that BioIVT has defined.
Sandwich-Cultured Human Hepatocytes (SCHH) –
