Drug Permeability Tools
Krishgen offers Permeability Assays from ReadyCell empower researchers with advanced tools to study drug permeability across various biological barriers. These in vitro models are designed to replicate human physiological conditions with high accuracy, making them an indispensable resource for drug discovery and development.
By leveraging these tools, researchers can generate predictive data on oral bioavailability, drug absorption mechanisms, and epithelial transport, ensuring smoother transitions from preclinical studies to regulatory approvals.

Cell-based assays with high flexibility and a ready-to-use system.
Highly predictive and useful in the in vitro to in vivo progression.
Worldwide shipments at room temperature thanks to our patented technology.
Why Use Permeability Assays?
- Predict Oral Bioavailability:
Evaluate how efficiently drug candidates are absorbed through the intestinal epithelium, a critical factor in oral drug formulation. - Understand Passive and Active Transport:
Differentiate between passive diffusion and transporter-mediated drug uptake or efflux, providing comprehensive insights into drug transport mechanisms. - Support Regulatory Requirements:
Generate high-quality permeability data aligned with guidelines from global regulatory bodies, including the FDA and EMA. - Optimize Drug Formulations:
Study permeability to identify compounds with ideal absorption profiles or to reformulate low-permeability drugs for improved performance. - Reduce Animal Testing:
ReadyCell’s in vitro models provide ethical alternatives, adhering to the 3Rs principle (Replacement, Reduction, Refinement) while offering reliable human-relevant data.
Applications of ReadyCell Permeability Assays
- Drug Discovery and Preclinical Development:
Screen compounds for their permeability and absorption potential early in the drug development pipeline. - Formulation Testing:
Evaluate the impact of excipients or delivery mechanisms on drug permeability and optimize formulations accordingly. - Pharmacokinetics and ADME Profiling:
Integrate permeability data into broader ADME studies for more accurate pharmacokinetic modeling. - Blood-Brain Barrier (BBB) Studies:
Use specialized models like PreadyPort BBB Kits to assess the ability of compounds to cross the blood-brain barrier, critical for CNS-targeted drugs. - Mucosal Drug Delivery Research:
Investigate drug transport across epithelial barriers like the oral, nasal, or pulmonary mucosa using relevant cell-based models.
CacoReady
Industry gold standard for intestinal permeability studies.
Features 21-day differentiated Caco-2 cells forming a polarized epithelial monolayer.
Applications: Passive diffusion studies, active transport assessments, and intestinal drug absorption prediction.
CacoGoblet
Combines Caco-2 and mucus-secreting HT-29 cells for a physiologically relevant intestinal model.
Applications: Drug absorption studies, anti-inflammatory drug testing, and barrier integrity evaluations..
PreadyPort
Simulates the blood-brain barrier using MDCKII cells.
Applications: Assess CNS-targeted drug permeability and transporter interaction studies.
Key Features of ReadyCell Permeability Assays
High-Quality Epithelial Models:
ReadyCell's kits utilize fully differentiated, polarized epithelial cells, such as Caco-2 and MDCKII, to mimic the structure and function of intestinal and other epithelial barriers.
Physiological Relevance:
Models like CacoGoblet Kits integrate mucus-secreting HT-29 cells, providing a barrier that closely mimics the intestinal environment, enhancing relevance to in vivo conditions.
Scalability and Convenience:
Available in formats suitable for high-throughput screening, the kits support both small-scale R&D and large-scale preclinical studies.
Innovative Shipping Technology:
ReadyCell’s patented Shipping Medium® ensures that kits arrive with viable, ready-to-use cells, eliminating preparation delays and maintaining experimental reliability.
ABSORTION ASSAY PROTOCOL
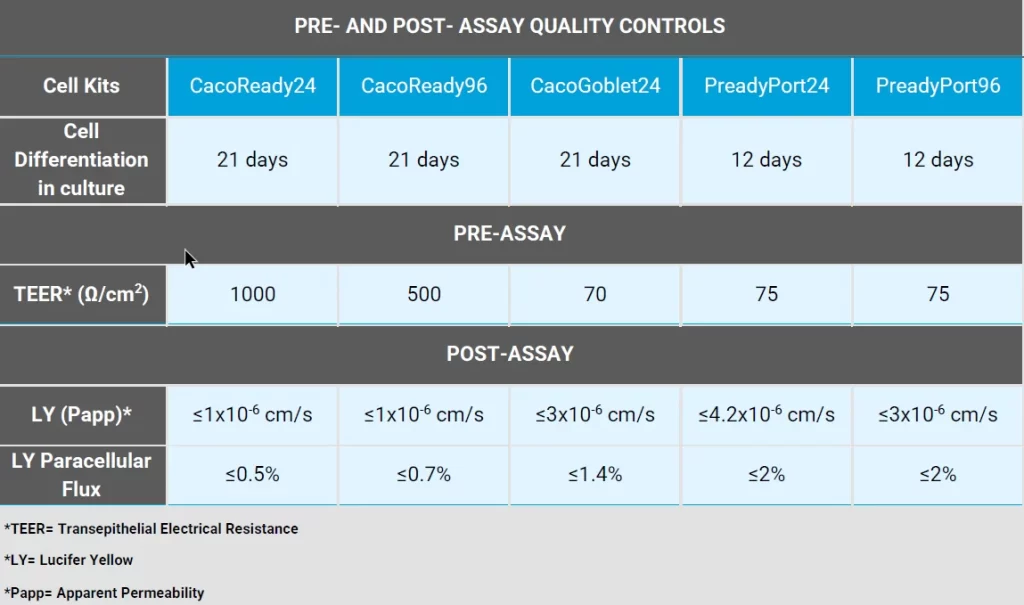
Reference values for Caco-2 permeability assay
If you want a comprehensive guide on conducting a Caco-2 permeability test procedure, check out this detailed protocol.
Frequently Asked Questions
Yes, we provide a concrete quality control for each batch so the customer can have a reference for the kit before and after shipment.
CacoReady and CacoGoblet are both, two cell-based kits to in vitro assay drug intestinal permeability. However, CacoReady only contains absortive epithelial cells (Caco-2 cells) while CacoGoblet is the result of coculturing Caco-2 cells and mucus-secreting cells (HT29-MTX cells), bringing it closer to the most physiological conditions.
Because they consist of different cell phenotypes, the barrier properties are also different being very tight for CacoReady with TEER values ≥ 1000 ohms x cm2 and looser for CacoGoblet (≥ 70 ohms x cm2), being the drug permeability assays for both models very consistent.
No, ReadyCell’s Shipping Medium consists of a semi-solid culture system specifically designed to preserve cells at room temperature (15-25ºC). This medium maintains a suitable physicochemical environment, keeping adequate moisture conditions for cellular homeostasis and forming a protective cushion that protects cell integrity and functionality during long-distance shipments up to seven days.

