BioIVT Cell & Gene Therapy / Complex Solutions
BioIVT offers high-quality human biological materials and tailored solutions to support research, development, and manufacturing in cell and gene therapy. BioIVT’s extensive portfolio includes mobilized leukopaks, Human AB Serum, and GMP-grade products, ensuring reliability and regulatory compliance for a wide range of applications.

Stock Availability

Wild Access of Donors

High Quality

BioIVT is the worldwide leader of biological Cell & Gene Therapy-products to life sciences and pharmaceutical companies.
Here’s what they have to say about working with us.
They are committed to providing high-quality CGT (Cell and Gene Therapy) solutions, offering specialized biological materials and services to support research, development, and manufacturing of innovative therapies. With a focus on reliability and scalability, we enable seamless advancement from discovery to clinical applications.
We Provide
- Customization of all products available
- Knowledgeable and attentive customer service team
- Products delivered in a timely manner, ready for use
- Unconditional product guarantee
- Saturday shipments available
- Same day collection, shipment of time sensitive blood samples, tissues and PBMCs
- Wide variety of biological products available
1. Human & Animal PBMCs:
These cells are manufactured with optimized methods to ensure a high-quality product with superior viability, purity, and functionality. Utilization of our cell products can yield a faster time-to-market as less time will be spent on sample preparation and leaving our clients to focus on more technically challenging endpoint assays.
Researchers can receive PBMCs and other fresh material less than 24 hours after collection.
These cell isolations can either be shipped fresh overnight for immediate use or cryopreserved for future scheduled work.
- PBMCs can be prepared from a variety of source matrices: vacutainer collection, buffy coat, leukopak and more
- Further processing performed, including RBC lysis, washing, and counting
- Aliquoting by cell count
Patient samples can also be prospectively collected to meet study-specific inclusion and exclusion criteria. All collections are conducted under IRB-approved protocols, and all donors sign informed consent forms where applicable. Samples are fully characterized and come complete with demographic and medical data, as recorded on case report forms at the time of collection.
BioIVT also offers bone marrow mononuclear cells (BMMCs) and cord blood mononuclear cells (CBMCs).

Human

Rat/Mouse

Rabbit

Monkey

Mini Pig

Hamster

Guinea-Pig

Dog
2. Leukopacks-Human:
Offering LEUKOMAX® leukopak products from both normal and disease state donors.
Our diverse donor pool consists of over 400 HLA-typed recallable, consented donors around the globe. All products are collected using strict IRB protocols at strategically located facilities under FDA and HTA guidelines. With complex therapies, the process is the product – so ensuring high-quality starting material is a critical first step in maximizing your results.
BioIVT’s new line of VivoSTART™ GMP-compliant Leukopaks and Plasma
derived/Off-the-clot Human AB Serum can seamlessly integrate into
your workflow.
- Collections performed in an FDA-registered, GMP-compliant center that meets 21 CFR 1271 specifications.
- Batch record documentation in compliance with FDA and HTA requirements
Maximize your GMP immune cell expansion with VivoSTART Human AB Serum to reach therapeutic doses faster.
We also offer mobilized leukopaks whereby donors are injected with G-CSF (FDA-approved). This drug stimulates the bone marrow to produce a large number of hematopoietic and progenitor stem cells and mobilizes them into the peripheral bloodstream where they are collected via leukapheresis.
BioIVT also offers both fresh and cryopreserved leukopaks from donors with confirmed autoimmune or infectious diseases, such as Diabetes, Rheumatoid Arthritis, Psoriasis, SLE or HIV, HBV and HCV.
The table provides LEUKOMAX Leukopak sizes and the percent of different cell subtype yield.
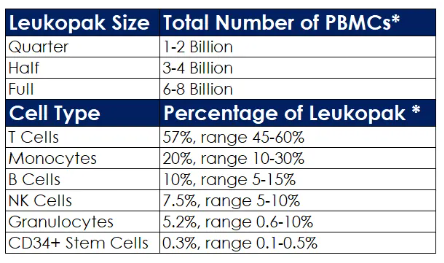
*Values estimated
Collection Process
Leukopaks are obtained through a process called leukapheresis. During this procedure, leukocytes are separated from whole blood with/without platelets with continuous or intermittent return of the RBCs and platelet- and leukocyte-poor plasma to a donor.
This blood-derived product contains a higher concentration of cells as compared to standard venipuncture collection methods or buffy coat products and is useful for those researchers looking for a large number of leukocytes from a single donor.
For more information on our collection process and cancellation policies, please see our Leukapheresis Collections page.
Select from available Leukopak products or contact us to discuss your needs as we are continually recruiting validated well-characterized recallable donors for your research needs.
3. Mobilized Leukopacks-Human:
BioIVT offers mobilized peripheral blood leukopaks containing a higher concentration of CD34+ cells from a single donor, and can provide them fresh within 24 hours to select locations or cryopreserved for convenient research scheduling.
Mobilized LEUKOMAX® Leukopaks
BioIVT offers mobilized leukopaks from healthy, IRB-consented human donors. These units deliver enriched concentrations of mononuclear cells, B cells, T cells, stem/progenitor cells, and dendritic cells compared to standard blood collection methods. Ideal for applications requiring high cell yields from a single donor.
Mobilization Process:
Donors receive G-CSF (Granulocyte-Colony Stimulating Factor), such as FDA-approved Neupogen®, to stimulate bone marrow production and mobilize hematopoietic and progenitor cells into peripheral blood.
Collection Method:
All leukopak units are collected using the Spectra Optia® Apheresis System, ensuring consistent quality and performance.
Features and Benefits:
- Customizable samples to meet specific protocol needs.
- IRB-approved donor collection protocols ensuring ethical sourcing.
- Comprehensive donor demographic data, including age, gender, smoking status, ethnicity, medications, and surgical history.
Product Details:
Mobilized units are provided as a single-unit bag.
Average Mononuclear Cell Yield: 2.3 x 10^10 cells.
Range: 0.9 – 5.6 x 10^10 cells.
Choose BioIVT for high-quality, mobilized leukopaks designed for research and therapeutic advancements.
Dosing Regimens Available:
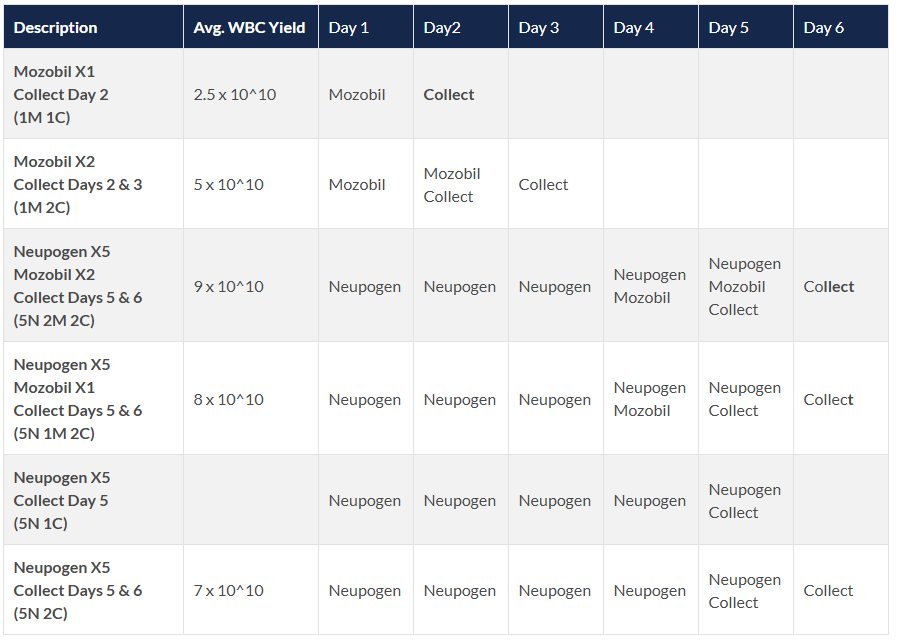
4. Human AB Serum- RUO & GMP:
Overview
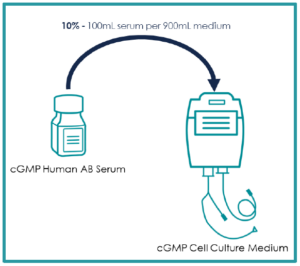
- Human AB Serum is a cell culture media supplement used to help expand cells for cell and gene therapy applications.
- Replacement to eliminate animal origin Fetal Bovine Serum (FBS)
- Collected from a single gender (male) and single blood type (AB) to reduce confounding effects and lot-to-lot variability
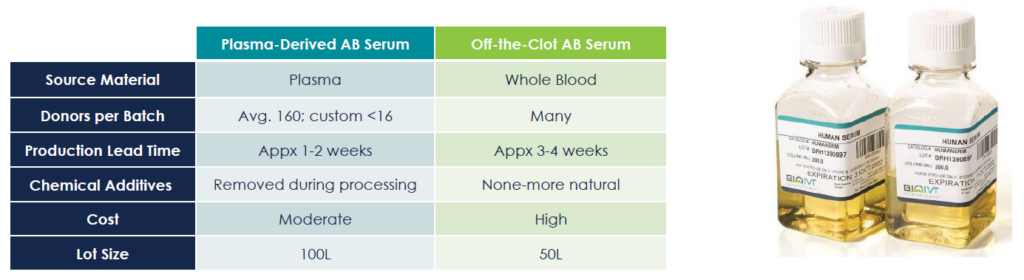
- Two kinds of serum available: Plasma-derived and Off-the-clot
Source Material
Derived from healthy male donors with AB blood type, Human AB Serum minimizes immunoreactivity due to the absence of blood group antibodies. Typically pooled from 150-250 donors, BioIVT also offers custom batches with fewer donors, including single-donor lots, to meet specific needs.
Production Methods
- Plasma-Derived Serum – Produced from donor plasma, defibrinated using thrombin to create serum.
- Off-the-Clot Serum – Extracted from naturally clotted whole blood, retaining growth factors and compounds for enhanced biological activity.
Viral Testing and Safety
Every batch undergoes rigorous testing for safety against pathogens, including:
- Hepatitis B, C, A (plasma-derived only)
- HIV 1/2, Syphilis, West Nile Virus, HTLV I/II, Chagas, TSE/BSE
- Parvovirus B19 (plasma-derived only)
Additional tests are available to meet regulatory standards.
GMP-Grade Serum
BioIVT’s GMP-grade Human AB Serum follows strict protocols with regulatory oversight for enhanced safety. Ideal for cell therapy manufacturing, it ensures compliance in material collection, processing, and quality assurance.
Optional Processing
- Sterilization – Filtered down to 0.1 μm for purity.
- Heat Inactivation – Disables complement activity without affecting growth factors.
- Gamma Irradiation – Provides viral inactivation for cell and gene therapy applications.
Applications
Human AB Serum is widely used in cell and gene therapy, especially for CAR-T cell expansion, improving therapeutic outcomes. It accelerates manufacturing timelines for patient-specific therapies and serves as a negative control in in vitro diagnostics.
5. Other immune cell products:
Manufactured with optimized methods to ensure a high quality product with superior viability, purity and functionality.
Utilization of our cell products can yield a faster time-to-market as less time will be spent on sample preparation, leaving our clients to focus on more technically challenging end point assays.
Isolated cell populations are ideal for researchers looking to perform:
- Flow Cytometry
- Functional Cell-based Assays
- Gene Expression Analysis
Cell subsets include T cell, B cells, NK cells, CD34+ cells and many other types. Clients can specify their preferred isolation method (positive/negative selection).
6. Other Cell Products:
Bone Marrow
BMMCs
CD34 HSCs
Cord Blood
Cord Blood CBMCs
CD34 HSCs
Spleen / Lymph Node / Tonsil
Splenocytes / Lymphocytes/ TMNCs
T Cells
B Cells
More About BioIVT
ADME 101: Drug Metabolism Studies – Metabolic Stability
Important Regulatory & Operational Considerations when Sourcing Cellular Starting Materials & Ancillary Products for Cell Therapy Development & Commercialization Expert Roundtable with Matthew Chorley, Dr. Parijat Jain & Wini Luty.
Important considerations should be taken in the selection of a manufacturer to source Leukocytes/Cellular starting materials and related ancillary products. Manufacturers must be GMP/GTP compliant and capable of sourcing RUO and GMP materials to allow an efficient path for ATMPs to move through the clinical pipeline and be approved for commercial use.
The FDA has promulgated a particular set of regulations, referred to as GTPs, that specifically address the need to procure and process tissues in a manner that avoids transmission of a communicable disease. GTPs and GMPs must be followed for cell-based advanced therapies or tissue-based therapy products.
Featured Products
Flyers and Product Guides
- Flyers
- Product Guides
- White Papers
- Application Notes
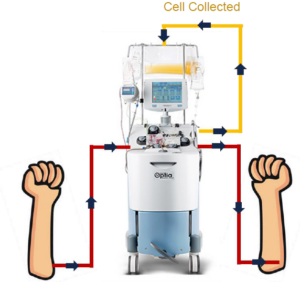
We utilize Terumo’s Spectra Optia® apheresis system for all leukapheresis products
BioIVT’s optimized collection protocols maximize MNC yields, providing more monocytes, lymphocytes, and CD34+ cells, while reducing red blood cells, granulocytes, and platelets
Human AB Serum Origin & Usage
Stock Availability in the Mumbai Warehouse
This stock may have discrepancies as it is not updated in real time. Please get in touch with our team before placing your final order.
PBMCs,
HUMANPBMC-0103664 - Human Mononuclear Cells (PBMC) 50X10^6 CryoStor CS10 (LMX ACD A) Cryopreserved
| Pack Size | Batch No. | Stock | COA |
| 50 Million Cells | HMN697428 | 11 vials each | HUMANPBMC-0103664-HMN697428 |
| 50 Million Cells | HMN697357 | 11 vials each | HUMANPBMC-0103664-HMN697357 |
| 50 Million Cells | HMN1272545 | 11 vials each | HUMANPBMC-0103664-HMN1272545 |
| 50 Million Cells | LS1174027C | 1 vials each | NA |
| 50 Million Cells | LS1174328B | 1 vials each | HUMANPBMC-0103664-LS1174328B |
| 50 Million Cells | LS1174801C | 1 vials each | HUMANPBMC-0103664-LS1174801C |
| 50 Million Cells | LS1175136E | 1 vials each | HUMANPBMC-0103664-LS1175136E |
| 50 Million Cells | LS1175435C | 1 vials each | HUMANPBMC-0103664-LS1175435C |
| 50 Million Cells | LS1175477C | 1 vials each | HUMANPBMC-0103664-LS1175477C |
| 50 Million Cells | LS1175330A | 1 vials each | HUMANPBMC-0103664-LS1175330A |
| 50 Million Cells | LS1176678B | 1 vials each | HUMANPBMC-0103664-LS1176678B |
| 50 Million Cells | LS1177461B | 1 vials each | HUMANPBMC-0103664-LS1177461B |
| 50 Million Cells | LS1177554C | 1 vials each | HUMANPBMC-0103664-LS1177554C |
| 50 Million Cells | LS2428714B | 1 vials each | HUMANPBMC-0103664-LS2428714B |
| 50 Million Cells | LS2428878C | 1 vials each | HUMANPBMC-0103664-LS2428878C |
| 50 Million Cells | LS2432037C | 1 vials each | HUMANPBMC-0103664-LS2432037C |
| 50 Million Cells | LS2433428C | 1 vials each | HUMANPBMC-0103664-LS2433428C |
| 50 Million Cells | LS8849991 | 1 vials each | HUMANPBMC-0103664-LS8849991 |
| 50 Million Cells | LS8848182B | 1 vials each | HUMANPBMC-0103664-LS8848182B |
| 50 Million Cells | LS1177485B | 1 vials each | HUMANPBMC-0103664-LS1177485B |
HUMANPBMC-0002021 - Human HLA typed PBMCs 10X10^6 CryoStor CS10 (LMX ACD A) Cryopreserved
| Pack Size | Batch No. | Stock | COA |
| 10 Million Cells | HMN621838 | 4 vials | HUMANPBMC-0002021-HMN621838 |
| 10 Million Cells | HMN621833 | 4 vials | HUMANPBMC-0002021-HMN621833 |
| 10 Million Cells | HMN621837 | 4 vials | HUMANPBMC-0002021-HMN621837 |
| 10 Million Cells | HMN621840 | 4 vials | HUMANPBMC-0002021-HMN621840 |
| 10 Million Cells | HMN621832 | 3 vials | HUMANPBMC-0002021-HMN621832 |
| 10 Million Cells | HMN621831 | 3 vials | HUMANPBMC-0002021-HMN621831 |
| 10 Million Cells | HMN621842 | 3 vials | HUMANPBMC-0002021-HMN621842 |
| 10 Million Cells | HMN621843 | 3 vials | HUMANPBMC-0002021-HMN621843 |
| 10 Million Cells | HMN621854 | 3 vials | HUMANPBMC-0002021-HMN621854 |
| 10 Million Cells | HMN697390 | 3 vials | HUMANPBMC-0002021-HMN697390 |
| 10 Million Cells | HMN621835 | 3 vials | HUMANPBMC-0002021-HMN621835 |
| 10 Million Cells | HMN621827 | 3 vials | HUMANPBMC-0002021-HMN621837 |
| 10 Million Cells | HMN621889 | 1 vial | HUMANPBMC-0002021-HMN621889 |
| 10 Million Cells | W462823001877-01 | 1 vial | HUMANPBMC-0002021-W462823001877 |
| 10 Million Cells | HMN621894 | 1 vial | HUMANPBMC-0002021-HMN621894 |
| 10 Million Cells | HMN621876 | 1 vial | HUMANPBMC-0002021-HMN621876 |
| 10 Million Cells | HMN621891 | 1 vial | HUMANPBMC-0002021-HMN621891 |
| 10 Million Cells | HMN621882 | 1 vial | HUMANPBMC-0002021-HMN621882 |
| 10 Million Cells | HMN621892 | 1 vial | HUMANPBMC-0002021-HMN621892 |
HUMANPBMC-0103644 - Human PBMC, gender unspecified, leukomax unit ACD A (50x10^6)
| Pack Size | Batch No. | Stock | COA |
| 50 million cells | LS1174328B | 1 | NA |
| 50 million cells | LS8848182B | 1 | NA |
| 50 million cells | LS1174801C | 1 | NA |
| 50 million cells | LS1175136E | 1 | NA |
| 50 million cells | LS2428878C | 1 | NA |
| 50 million cells | LS2432037C | 1 | NA |
| 50 million cells | LS1175330A | 1 | NA |
| 50 million cells | LS2433428C | 1 | NA |
| 50 million cells | HMN697357 | 11 | NA |
| 50 million cells | LS1176678B | 1 | NA |
| 50 million cells | HMN1272545 | 11 | NA |
| 50 million cells | LS1175435C | 1 | NA |
| 50 million cells | LS1177485B | 1 | NA |
| 50 million cells | LS2428714B | 1 | NA |
| 50 million cells | LS1177461B | 1 | NA |
| 50 million cells | LS8849991 | 1 | NA |
| 50 million cells | LS1175477C | 1 | NA |
| 50 million cells | HMN697428 | 11 | NA |
| 50 million cells | LS1174027C | 1 | NA |
| 50 million cells | LS1177554C | 1 | NA |
HUMANPBMC-0001989 - Human PBMC, gender unspecified, leukomax unit ACD A (5x10^6)
| Pack Size | Batch No. | Stock | COA |
| 5 million cells | 172010281 | 1 | NA |
| 5 million cells | HMN697400 | 1 | NA |
| 5 million cells | LS1162110C | 1 | NA |
| 5 million cells | LS1171059A | 1 | NA |
| 5 million cells | LS1172398A | 1 | NA |
| 5 million cells | LS1172926C | 1 | NA |
| 5 million cells | LS2417349B | 1 | NA |
| 5 million cells | HMN806447 | 1 | NA |
| 5 million cells | LS1170556B | 1 | NA |
| 5 million cells | HMN697391 | 1 | NA |
| 5 million cells | LS1171078A | 1 | NA |
| 5 million cells | 198002127 | 1 | NA |
| 5 million cells | HMN621848 | 1 | NA |
| 5 million cells | LS1160807C | 1 | NA |
| 5 million cells | HMN806451 | 1 | NA |
| 5 million cells | LS1175540 | 1 | NA |
| 5 million cells | HMN621816 | 1 | NA |
| 5 million cells | HMN621877 | 1 | NA |
| 5 million cells | W462823003213 | 1 | NA |
| 5 million cells | LS1173040A | 1 | NA |
| 5 million cells | LS1175261 | 1 | NA |
Human AB Serum
HUMANABSRMC-HI-X1 - HUMAN AB SERUM PLASMA - off the clot, non filtered, heat inactivated
| Pack Size | Batch No. | Stock | COA |
| 100ml | HUMANABSRMC-HI-X1 | 50 | NA |
HUMANABSRMC-1 - HUMAN AB SERUM PLASMA - off the clot, 0.1uM filtered
| Pack Size | Batch No. | Stock | COA |
| 100 ml | HUMANABSRMC-1 | 50 | NA |
HUMANABSRMCG-1 - HUMAN AB SERUM PLASMA - off the clot, 0.1uM filtered, GMP
| Pack Size | Batch No. | Stock | COA |
| 100 ml | HUMANABSRMCG-1 | 50 | NA |
Other Serum and Plasma
HUMANSRM1701138 - Human Serum Complement Preserved Male
| Pack Size | Batch No. | Stock | COA |
| 100 ml | HMN445309 | 1 | NA |
| 100 ml | HMN445307 | 1 | NA |
| 100 ml | HMN445308 | 1 | NA |
HUMANSRMP2N-25 - Human Serum Pooled Gender
| Pack Size | Batch No. | Stock | COA |
| 25 ml | HMN427402 | 1 | NA |
HP1000.HP+ - Individual male human plateable pellets, AMY 5 million cells
| Pack Size | Batch No. | Stock | COA |
| AMY 5 million cells | H1220 | 1 | NA |
| AMY 5 million cells | H1443 | 1 | NA |
| AMY 5 million cells | H1441 | 1 | NA |
Need More Information?
The Krishgen support team strives to provide swift responses and resolution to your queries. Get in touch for a technical question, product query or quote request.